Infection Detection Bandage
Team: anonymous MIT student [CM], Tiffany Yuh, Tiffany Guo, Elina Pradhan and anonymous MIT student [BG].
This content is presented courtesy of the students and used with permission.
- Problem Statement
- Lotion to Detect Infection?
- Bandaid to Detect Infection
- Nitrite as a Byproduct of Bacteria
- Other Biomarkers for Infection
- Griess Reaction
- Realization about Nitrites
- Ordering it… Some Versions Very Expensive
- Different Color for Detection
- Mitch Sanders – ECI Biotech
- Mitch Sanders – Patents and IP
- Aquarium Nitrite Kit
- Regular Re-dressings? Send them Home with a "Booklet" of Bandages
- Constant Monitoring for Infection – How to Use the Bandage
- Tegaderm™ to the Rescue, but Not Quite
- Clear Tape Packaging
- Team POP Video
- Comments on Video
- Preliminary Testing
- Detectable Nitrite Concentration
- Threshold Concentration for Color Indicator to Show
- Bacterial Testing
- Will it Work Dried?
- Dried Reagent Packaging Methods
- Wet Reagent Packing Methods
- Methods for Keeping Griess Reagent Away from the Wound
- Putting the Demo Together
- Reflections on the Museum
- Further Experimentation
- Burden of Disease
- Talk with Joseph Chung, Founder of Thalas
- How Patient Would Use Bandage
- Delivery of Reagent
- Comment on MIT Museum Poster Expo
Problem Statement
by Tiffany Yuh
In developing countries such as Nicaragua, the lack of sterile equipment and operating areas, as well as proper standards of care, is a significant issue, frequently causing post-operative infections. People often live far away from health clinics, making it time-consuming and costly for patients to report back for post-operative care. The result is often a worsening of the infection, which can also cause a financial drain on the individual and his or her family, as it may take away from their quality of life and ability to work. We hope to create a device that will allow for early detection of infection in the wound, giving a clear indication to the patient that an infection has developed without the need for a clinician's diagnosis, and notifying the patient early on before the infection gets any worse.
Lotion to Detect Infection?
by Elina Pradhan
One diagnostic method could be to have a lotion or a liquid of some kind that has the detection mechanism in it. Colorimetric detection could be the simplest method of detection.
Idea: a clear or a white lotion that will turn into a different color when it detects any infection.
Bandaid to Detect Infection
by [BG]
One of the first decisions that we had to make was the size of the bandage we were going to design. Naturally, a Band-Aid was one of the first possibilities. They are recognizable, commonly used, and easy to use. Therefore, a slightly altered Band-Aid that provides infection detection would likely be easy to disseminate. The major problem with Band-Aids, however, are their size. Unless a wide variety of sizes were designed, it would be difficult to address post-operative wounds of varying size. This is the primary reason that we opted to create a bandage material that could be tailored to fit the appropriate wound rather than using a Band-Aid, which typically is used for small cuts and scrapes instead of for larger post-operative wounds.
Nitrite as a Byproduct of Bacteria
by [BG]
Bacteria utilize two major metabolic pathways—aerobic and anaerobic. When oxygen is present, bacteria will use oxygen as the final electron acceptor during the oxidation of chemical energy sources such as glucose. However, when oxygen levels are decreased bacteria will undergo anaerobic metabolism whereby they use nitrate (N03) as the final electron acceptor. This reduction of nitrate produces nitrites (NO2). Bacteria may also further reduce nitrite to ammonia (NH4). In exploring mechanisms of infection detection we realized that these metabolic byproducts (NO2 and NH4) could be potential biomarkers for infection. This led us to using the Griess reagent which is a well known reaction that detects nitrites. Conveniently it also indicates the presence of nitrite through a colorimetric mechanism which is exactly what we were looking for in our detection mechanism.
Citation: Cole, J. "Nitrate Reduction to Ammonia by Enteric Bacteria: Redundancy, or a Strategy for Survival During Oxygen Starvation?" FEMS Microbiol Lett 136 (1996): 1-11.
Other Biomarkers for Infection
by Tiffany Guo
Because we are trying to detect infection, we had to do some research on what exactly happens in an infection, and what we could possibly use to detect one. A biomarker is defined as a quantifiable chemical/protein or biological factor that can be measured as an indicator of a disease or condition, in this case, infection. Some specifications and criteria that we identified for our biomarkers: accurate, safe(non toxic to body), safe (non-toxic to environment), sustainability (maintains molecular integrity) under harsh environmental conditions, and cheap.
The challenge with finding our biomarker is that we need to detect infection before it gets to the obviously visible stage (redness, swelling), or else our detection method is no better than a visual nurse. After looking up several articles online through Wikipedia and PubMed, one possible biomarker is chemokines. Chemokines is a class of proteins that are one of the first recruited to the infection site; they are used to direct white blood cells to the site of infection so should be a relatively early signal. However, we still don't have very specific knowledge about the time scale of infection to chemokine secretion, chemokine secretion to white blood cell recruitment, and white blood cell recruitment to visible infection. Another potential difficulty is that chemokines are most likely detected via antibodies, which require specific conditions to remain stable and are also expensive. We may have to look into other detection mechanisms, but chemokines may be an early indicator of infection.
Griess Reaction
by Elina Pradhan
I found a reaction system to detect nitrates. We can use nitrate reductase in presence of NADH to reduce nitrates to nitrites, and use Griess reaction to detect nitrites. The reaction between sulfanilamide and nitrite in presence of water gives a product which reacts with azo-dye to give off a bright pink color.
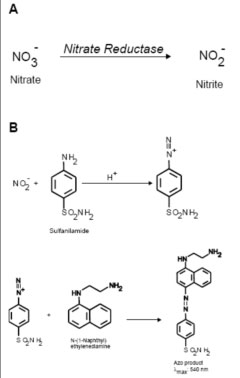
Nitrate reductase reaction. (Courtesy of BioTek Instruments, Inc. Used with permission.)
Figure from Held, Paul. "BioTek PowerWave 200 Microplate Spectrophotometer: Nitric Oxide Synthesis." BioTek Application Note, February 7, 2001.
A couple of limitations: the reaction needs water (proton donors), so our system should have some moisture
Realization about Nitrites
by Elina Pradhan
Upon further research, found out that nitrites, not nitrates, are the byproducts of many species of bacteria. This reduces the number of the reactions that need to work for bacteria detection. Now we do not need nitrate reductase, and we can work only with Griess reaction system.
Ordering it… Some Versions Very Expensive
by Elina Pradhan
Now that we had a biomarker and a detection system planned out, we started searching online for asssay kits. Nitrate reductase was pretty cheap at 40 dollars per vial including shipping, but we had to search for a while to find an affordable Griess reagent kit. The total cost for the Griess reagent system was 87 dollars, and to our luck, it came with a nitrite standard, which we would use to test our prototype.
Different Color for Detection
by Tiffany Guo
Currently, the Griess reaction utilizes an "azo dye" which turns hot pink when reacted with nitrate. The benefit of a colorimetric test is that it is very simple and straightforward to "diagnose" presence of a signal. While blood does look different from the hot pink color made in the reaction, it may be even more user friendly to use a color that is unnatural: the signal cannot be mistaken as anything else, and anything else cannot be mistaken as the signal. Also, when we were considering having a clear bandage, we wanted to find a color that would contrast w with the skin. We brainstormed all different colors, and decided on either blue or green. Colors in the warm family (red, orange, yellow) were not considered good colors because they would be too close to skin tone. While it may be ideal to have blue or green be the detection color, there needs to be at least some chemical reaction, if not a different type of azo dye, in order to change the color of the reaction. While I don't know a ton about this specific colorimetric test, from what I understand, dyes change color because of a simple chemical reaction that changes the bonding and electron chemistry of the molecule. Either choosing a different dye or altering the azo dye shouldn't be impossible, but it will probably take some consultation with others outside of the group. However, this consideration is lower priority; we have to make sure that our detection system works before we start trying to change the color of it.
Mitch Sanders – ECI Biotech
by Tiffany Guo
Today, Jose referred us to Mitch Sanders of WPI, who started his own company called ECI Biotech. The company is based around creating detection systems for simple, sensitive, and accurate diagnostic and therapeutic systems for a variety of applications (feminine care, wound care, oral care, vision care, food safety, hospital infection control). Through the Web site, we learned that ECI Biotech has two main products: ExpressDetect (diagnostic system) and ExpressProtect (therapeutic system). ExpressDetect is almost exactly what we've been looking to develop, a color indicating bandage when an infection is present. The specific biology wasn't available on the website so we'll have to dig deeper to find out what the exact biology is. The other portion is ExpressProtect that will actually take several chemical measures to reduce infection and inflammation while not harming host enzymes. It looks like this is not yet a feature that is embedded into the ExpressDetect bandage. However, a consideration that our group had made in brainstorming was to have, upon detection, release of some antibiotics, so that the infection would be treated without even having to go to a doctor.
We will email him for consultation, hopefully he can be of some help!
Mitch Sanders – Patents and IP
by Tiffany Guo
After looking online more about Mitch Sanders, it looks like he has about 21 patents in the area. Currently, he also has a very broad patent application in "substrate detection." However, the patent is not one that covers "the idea of using a bandage to detect infection" (that's probably a difficult patent to make robust anyways). This also makes him somewhat of an intimidating consult because he does have so much IP in the area, he may see us as a competitor, and may be unwilling to collaborate. We'll see how it goes.
Glancing through the patent, basically, the idea is that the mechanism or system has two different color molecules connected to each other (probably by polymer or peptide), and when the correct enzyme (specific to infections) comes along, it will cleave the peptide revealing just one color molecule, revealing that color. The mechanism of action is definitely distinctly different from ours, so in that case, our idea would not infringe on his current patent application. Also, it's likely that his bandage would not be cheap compared to our solution. In this light, he may not be an expert in terms of helping us hone the reaction of our bandage, but would have a good idea about the time-course of infection.
Aquarium Nitrite Kit
by Tiffany Yuh
Ryan brought up an interesting concept—those with fish as pets often test the aquarium water using nitrite testing kits. Nitrites are a toxic waste material produced by nitrifying bacteria. Nitrite levels in the water significantly affect the health of the fish, as nitrites reduce the ability of their red blood cells to carry oxygen, leading to their suffocation. An online search shows an abundance of these nitrite kits for aquariums. Petco sells a kit by Aquarium Pharmaceuticals for a mere $5.99 which uses colorimetric analysis to determine nitrite levels. This would be interesting to look into!
Nitrite Test Solution kit by Aquarium Pharaceuticals. (Courtesy of Mars Fishcare, Inc. Used with permission.)
Do Patients Change Bandages Everyday?
by [CM]
One of those important questions when dealing with a bandage solution is how often the patient changes their dressing. We spoke with an ER doctor, Dr. Leo Celi, and he suggested that bandages stay on for the length of the healing process. But what if they need to shower? The patient is instructed to cover the bandage with plastic, and to not get it wet. All this is the standard of care, but may not be so realistic for developing countries. In that case, we needed to add to our protocol the allowance of at least a few bandages and patient or caretaker training to administer the testing and re-dressing.
Regular Re-dressings? Send them Home with a "Booklet" of Bandages
by [CM]
Now what if the patient needs to change their bandage everyday, or every other day? We came up with a booklet idea, where there would be a removable bandage on a page for each day of care. The first page would have instructional photos teaching the patient how to re-dress their wound, and a color square to compare the color of the bandage if they believe an infection is present. I mocked-up a prototype, seen here:


"Booklet-style" packaging for a multiple day supply of bandages.
Nice idea if we can get the Griess reagent to dry on a bandage.
Constant Monitoring for Infection – How to Use the Bandage
by [CM]
If the bandage stays on for the length of the healing process, and the Griess reagent offers a 24/7 constant monitoring service, how does the patient use the bandage? The outer dressing–the adhesive, in our model, packing tape–is clear so that the patient or caretaker can see the gauze touching the wound. The gauze is impregnated with the Griess reagent, and will turn pink during an infection. This pink color is easily visible on the white background of the bandage and through the clear adhesive. If the wound is easily accessible, then the patient will primarily monitor the bandage for a color change, checking visually at least once an hour. If the wound is inacessible by the patient, a caretaker (usually a spouse or family member) will check the bandage.
Tegaderm™ to the Rescue, but Not Quite
by [CM]
Tegaderm™ is a great product, if you're willing to pay the $5 cost per bandage. It offered the perfect set of design specs for the bandage: transparent, breathable, sterile, nicely packaged for mobility and easily cut-to-size. We initially thought that if we could impregnate a material like Tegaderm outright, then we've got a winning solution. Even better, if we could silk-screen or print the reagent onto the material, we could spell out messages, like "You have an infection." or "Contact a doctor." Either way, Tegaderm could replace our packing tape as the choice dressing that secures the Griess-soaked gauze, but it's simply too expensive of an option.

A Tegaderm sample.
Clear Tape Packaging
by [CM]
What it comes down to is that the patient or caretaker needs to see the gauze to monitor the color. In the real setting, we'll need to opt for something locally available and sterile: clear plastics, for instance. In our demos, we tried two materials: clear packaging tape, and clear plastic packaging:
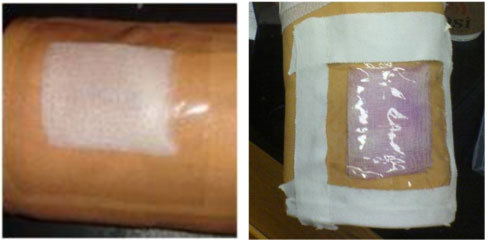
Two prototype bandages.
To use the clear plastic, we cut it to size, overlaid it on the wound, then used commonly available surgical tape to secure the edges against the skin. This solution worked nicely, because it was easily cut-to-size. In the second demo, we used the packaging tape. The Griess reagent-gauze combo was pressed against the tape, which was then dressed on the wound. We found that the tape itself 1) does not stick well to skin for long periods, and 2) loses it adhesive properties once wet (here, from the Griess reagent and nitrite solutions we used during the demo).
Team POP video
by Tiffany Yuh
Here is a video we've created:
Free downloads:
iTunes U (MP4 - 6.1MB)
Internet Archive (MP4 - 6.1MB)
It depicts a possible scenario of a patient getting an infection, and provides background on the problem we are attempting to solve. We suggested a preliminary model that consists of a clear bandage (similar to Tegaderm) without gauze that turns green upon signs of early infection. This tape would come on a roll, and is impregnated with the reagent. It is also clear to allow for monitoring of the wound without removal of the bandage.
Comments on Video
by Elina Pradhan
The most common comment I received was regarding the pink color. Many people observed that pink is very close to the color of blood, and the device would be better if the color was blue or green or violet instead of pink. This would require us to search for another detection mechanism.
Some people were curious about the lower limit of the detection system. As we have observed before, this should be the next big step in our device design. We should conduct experiments on different species of bacteria and find out the lower detection limits of our reaction system.
Preliminary Testing
by Tiffany Yuh
On Ses #25, we performed preliminary testing using the Biotium Griess Reagent Kit that came in over the weekend. The kit included Griess reagent, as well as a vial of standard nitrite solution. This first test served mainly to give us a general sense of if the test worked, and what it looked like. We soaked a piece of filter paper with Griess reagent, and then placed a few drops of diluted nitrite solution (~1/2) onto it. The reaction was almost instantaneous, resulting in a bright pink color. We were excited to see the successful reaction and color change, but were concerned about the viability of using this reaction because of the lack of safety data about Griess reagent, as well as the need to keep the reagent cold and away from the light.

Adding Griess reagent to filter paper.
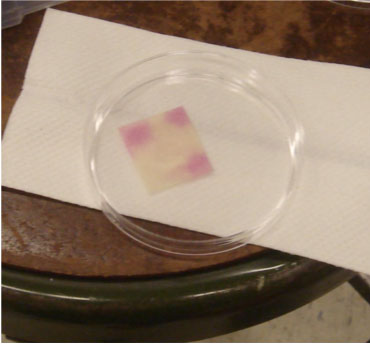
Result.
Detectable Nitrite Concentration
by Tiffany Yuh
We decided that it was important to test for what levels of nitrite were detectable by our Griess reagent. Therefore, we created eight different dilutions of our standard nitrite solution—1/2, 1/4, 1/8, 1/16, 1/32, 1/64. We added Griess reagent to absorbent pads (typically used in petri dishes to soak up bacteria media) and then dropped our diluted nitrite dilution onto each pad. You can clearly see a progressive dampening of the bright pink color as the concentration of the nitrite solution decreases. We concluded that the 1/16 dilution was the point at which Griess reagent could still detect the presence of nitrites. The further dilutions that you see in the petri dish on the right do not look significantly different from the control.
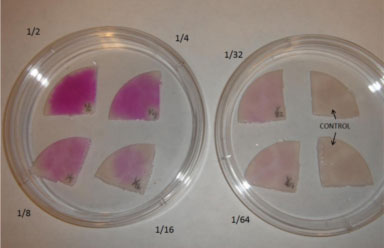
Color variations by nitrite level.
Threshold Concentration for Color Indicator to Show
by Tiffany Guo
One of the difficulties of the colorimetric tests now is that it's a gradient. For a user, it may be difficult to know if the pink they are seeing is just background pink color or if the bandage is indicating infection. The less judgement required in this situation the better, it reduces the chance of misdiagnosis: you want the bandage to be diagnosing infection, not the patient. At first, it seemed almost impossible chemically to make the reaction such that it will only indicate past a threshold level. However, after thinking some more, we realized that pregnancy tests do that, they give a very binary answer, "yes" or "no" based on the presence of follicle stimulating hormone. I think the pregnancy test is more based on the presence or absence of FSH (whereas our bandage would be looking for nitrite above a certain level), but it still has a very clear color signal at the user end of the device. Interestingly, there is not a lot of easily available information online that talks about the chemical reaction aspects of a pregnancy test. Like changing the indicator color in the reaction, we know this is theoretically possible, and we will need to have further consultation in order to implement the specifics. This consideration is also lower priority at this point because we need to first design a reliable early detection mechanism before making user based modifications such as these.
Bacterial Testing
by Tiffany Yuh
On 2 days after Ses #25, we attempted a test using bacteria. A plate of E. coli was borrowed from the nebulizer team, and a swab of colonies was taken up and transferred to an absorbent pad. Griess reagent was then added directly to the transferred colonies. However, no reaction seemed to occur. The slight pink color you see below is actually the muted pink color of the Griess reagent itself. The reason for the lack of reaction is unclear—it was noted that the bacteria might have been dead, as the plate had been sitting out for a while. Another possibility is that the E. coli did not actually produce detectable levels of nitrite.
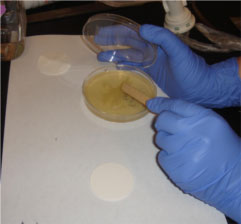
Step 1. Picking up the E. coli.

Step 2. Transferring to the pad.
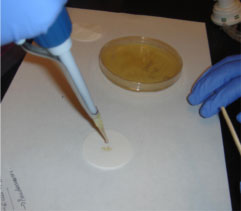
Step 3. Adding the Griess reagent.
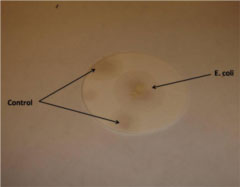
Result
Will it Work Dried?
by Tiffany Guo
When we were talking through ideas about our bandage, we thought it would be a good set up to have the reagents dried on the bandage ready to be used. With wet reagent, we would have to make considerations such as how to package the bandage and how to make sure the reagent doesn't get into the wound such that it is painful or harmful to the user. So we did a test to see if the reagent would work after being dried.
We dropped the Griess Reagent on absorbent pads and let them dry for 24 hours (while covered lightly with paper or parafilm). After dropping reagent on the pads, it was slightly pink. When we came back to look at the dried pads after 24 hours, the pinkish color was noticeably darker; however, the important thing to figure out is whether or not the difference in color is significant. We did three tests:
- Control: drop PBS
- Experiment 1: 1/4th dilution of stock solution, 0.25mM nitrite
- Experiment 2: stock solution, 1mM nitrite
Experiment 1 showed no noticeable change in color. Experiment 2 showed a noticeable change in color, but possibly not as drastic as we would like. We think that the reagent may need to be wet to work well, more dampness than it may get through the wound’s blood. We will either need to make changes where we add water to the reagent afterwards, or consider wet reagent packaging/distribution methods.
Dried Reagent Packaging Methods
by [CM]
The Urine Dipstick is a great example of how Griess reagent can be dried onto a material to extend its shelf life. I tried to research the manufacturing method, to no luck. However, for our purposes, the procedure is to make a bulk batch of reagent, apply (eg. via eye droppers) to the gauze squares, and then store the squares in a cool, dry (this is key) place away from strong sources of light. This way, the reagent does not degrade while it dries. Once the gauze squares are ready, they can be either applied directly to wounds, or packaged for later use. I can imagine this on an industrial scale, in clean rooms like they prepare medical instruments at Johnson & Johnson manufacturing plants: teams of workers using machinery to package the bandages in such a way they are both sterile and high-quality. The gauze packaging itself will need to keep moisture from the bandage, and protect it from light. I believe the common packaging methods for gauze would do just fine.
Wet Reagent Packing Methods
by Tiffany Guo
There were a couple different options brainstormed for how to package a system that uses a wet reagent system.
- Blister pack – We could have the doctor or healthworker use the same gauze that they normally use and moisten the gauze with a blister pack of reagent, and then cover it completely with clear medical tape. This would be extremely easy to package, just package a small volume of reagent.
- Alcohol wipe package – We could have already moistened gauze/pads in a moisture tight packaging (like alcohol wipes) that the user could just rip out of the package, and tape onto the wound with clear medical tape. The benefit of this form of packaging, is that you know that the gauze is evenly and adequately moistened, unlike in the case of the blister pack, where the user may not adequately moisten all portions of the gauze. It is every so slightly more complex to manufacture, but should not be very difficult.
In these cases, because we would want to keep the reagent wet, clear medical tape (quite common) would be used to tape the gauze pad over the wound, as well as to cover the gauze pad to keep the reagent from evaporating. The medical tape would need to be clear so that the user could see if ther was any color change. Another consideration is to make sure that the reagent (or at least too much of it) goes into the wound, as it could be painful. Many diapers have a film over the absorbent pad that "wicks moisture away from the body" keeping moisture in the absorbent pad but not letting any back towards the skin. We could probably have a similar film covering our absorbent pad to keep as much reagent away from the wound as possible.
Methods for Keeping Griess Reagent Away from the Wound
by [BG]
Griess reagent has "unknown" toxicity, so while it is likely safe, we can’t be sure without long clinical trials and OSHA clearance and the like. To get around this problem, it is easier (and cheaper) to develop a solution that does not allow Griess reagent to contact the skin. The film over the absorbent pad in a diaper (mentioned above) is a great solution to keep the Griess reagent in the gauze, if we choose to have a constant monitoring system where the reagent is deposited in the bandage. A second approach is to have the reagent completely separate from the bandage itself. This is how to do it: wound irrigation (wetting with saline) is a common practice for nurses when cleaning wounds–in fact, some wounds need to be irrigated anyways. The saline comes in small, cheap, disposable plastic vials the nurse carries. The saline would be poured into the wound, then a new piece of gauze could be used to pat the wound dry. This would wick up the water and any bacteria onto the gauze. The Griess reagent could then be dripped onto this dampened gauze, and monitored for a color change. The saline is required as a source of protons for the Griess reaction.
Putting the Demo Together
by [CM]
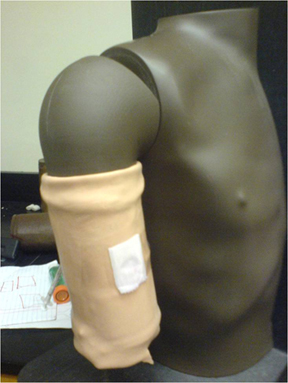
We had two demos at the MIT Museum. Both used a dummy torso and fake upper arm from the TB delivery kits in D-lab. For the smaller demo, we mocked-up the bandage on the arm, and simply slid the arm onto the metal rod in the torso. Voila. Demo number one.
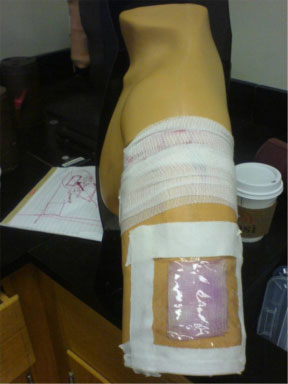
Bandage mockup for Demo #1
The larger demo (below) took some tinkering. The original arm was soft foam, and would not do. I removed the long threaded rod that held the arm on, which required taking apart the shoulder on the dummy torso to reveal the butterfly nut at top. I then poked a hole in the bottom of the arm, and threaded the rod through the center of the arm. Re-attaching this to the dummy torso gave us a quick arm transplant. Next, I cut the skin in the back of the arm, and threaded through a long, small capillary tube, and hid the mouth near the fake open wound. I connected the other end of the tube to a plastic syringe (using some serendipitous piece of plastic that came with the syringe–which I squeezed into the flexible capillary tube). It was detachable, which allowed us to fill the syringe with a nitrite solution, re-attach, then pump into the wound behind the scenes (literally, behind the arm, out of sight).

Bandage mockups for Demo #2, showing Griess reaction
During demos, a Griess-soaked bandage was applied to the arm over the wound, we injected the nitrite solution, and within seconds, the bandage changed to the typical pink color. It worked!
Reflections on the Museum
by [CM]
Amit mentioned we could use a baby wipe model for packaging the reagent: similar to the alcohol wipe model, but we would have multiple wettened gauze pieces in a single package. While disinfectants like in a baby wipe will sting if applied in a wound, the packaging method is appropriate for multiple uses. This kind of package might be sent home with the patient in place of the booklet of bandages (see postings given below).
by Elina Pradhan
The most common comment I received was regarding the pink color. Many people observed that pink is very close to the color of blood, and the device would be better if the color was blue or green or violet instead of pink. This would require us to search for another detection mechanism.
Some people were curious about the lower limit of the detection system. As we have observed before, this should be the next big step in our device design. We should conduct experiments on different species of bacteria and find out the lower detection limits of our reaction system.
by Tiffany Guo
The museum was one of the best forms of feedback/review. We’ve been working on the project for awhile, and made a lot of considerations, but it was a great way to hear a lot of different viewpoints and feedback for more ideas. I was also surprised by the amount of positive feedback that we got; I guess when you’re working on a project or idea for awhile, and almost all of your ideas are substantiated by information you’ve found on Google, then it’s difficult to know how novel or reasonable your idea will seem to other people. We had a number of people stop by our table and got great questions and suggestions. We had been struggling to figure out exactly how much nitrate was produced by bacteria in a bacterial infection, we couldn’t find it online, and even if we did have access to bacteria (which we’re working on getting through Amit) we weren’t sure how to accurately test it. One person suggested that we take samples of blood, and then add in small amounts of bacteria, incubate it, and test our bandage on those blood samples. We would need access to a lab that could do these kinds of experiments, but it seems to make sense and be relatively simple to do.
Further Experimentation
by [CM]
If we send a team back to Nicaragua, we can do an in-depth assessment of their standards of wound care: interview doctors, nurses, local health workers, and especially a variety of patients. We need to find out what is the standard practice, and what actually happens, then try to identify what shape our solution should take based on the data we collect. We could also test one or more of our solutions on real patients and their wounds, albeit one where the Griess reagent does not contact their skin. For instance, I suggest irrigating the wound, pat dry with sterile gauze, then dripping wet Griess reagent onto the gauze. An issue we would run into is keeping our Griess reagent fresh. Another simple solution would be to simply use the Griess reagent square on urine dipsticks: bring a bottle of the dipsticks to the point of care (hospital, clinic or patient home), irrigate the wound, pat dry with gauze, and then press a dipstick into the wet gauze. This would solve the shelf life and storage issues.
by Elina Pradhan
- Tests on Bacteria: Lower detection limit, Species of bacteria detected
- Toxicity assays: Cell toxicity assays, Animal testing for hazards related to the Reaction system
by [TY]
There is a lot to be done in terms of further experimentation. It would be particularly useful to perform some tests with bacteria. We could grow different concentrations of nitrite-producing bacteria and perform the Griess reaction upon these samples. Then, we would be able to determine what bacteria levels are necessary for a positive result on our colorimetric assay. Along with these experiments, we would need to research how bacteria levels correlate with the progression of an infection. Without this experiment/research, we are unable to correlate nitrite levels to the stage of an infection.
Burden of Disease
by [BG]
At the University Hospital of Leon, Nicaragua, a study was conducted to determine the effect of preoperative antibiotic prophylaxis on the prevention of post-operative infections. The controls which didn’t receive that antibiotic prophylaxis showed a 15% infection rate. We use this value as a measure of our burden of disease.
Citation: Disseldorp, J., E. J. M. H. Slingenberg, A. Matute, E. Delgado, E. Hak, and I. M. Hoepelman. "Application of Guidelines on Preoperative Antibiotic Prophylaxis in Leon, Nicaragua." Netherlands Jour Med 64 (2006): 411-416.
Talk with Joseph Chung, Founder of Thalas
by [BG]
At the D-lab poster session I spoke with Joseph Chung, the founder of Thalas—"the world's first and leading socially conscious umbrella organization." Initially he gauged the project by asking a few questions about the design theory and how we planned to proceed. I was straight-forward with him and explained that we unsure seeing as many of the group members would be moving on to take on different classes, jobs, and/or projects. Anyway he was incredibly interested in our idea and would be interested in talking with us further about marketing the idea in the U.S. and then using any funds to introduce the bandage in the developing world. However, he said he’d only be willing to talk if we were "serious." He was a young guy but said he had already started 5 companies. He also stressed that Thalas is a very socially-aware company that advises executives and people like us on projects to improve the world through many different ways. And last but not least, he mentioned that Thalas had a $20 million fund.
How Patient Would Use Bandage
by [BG]
After an operation, the doctor would provide the patient with the specialized bandage and extras (depending on the expected healing time for the wound). He would instruct the patient or a caretaker on how to properly replace the bandage. He would instruct the patient to return if the patient turns bright pink.
Diagram of how patient would use bandage (PDF)
Delivery of Reagent
by [BG]
There are a few issues with using the Griess reagent: (1) it requires an aqueous environment to function properly, (2) it doesn’t function after it is dried, and (3) it has a short shelf life when it is not stored properly. This poses obvious issues for our designed detection device. It is necessary to either develop a way of storing Griess reagent in the bandage such that it will function for a longer time or to change how we use the Griess reagent. Potential solutions include irrigating the wound regularly and using the Griess reagent to test the washing fluid for nitrates ex vivo and using a baby-wipe type of cloth that is moist with Griess reagent (and stored properly) to clean the wound regularly. This means that infection detection would depend on the patient regularly cleaning his or her wound.
Comment on MIT Museum Poster Expo
by [BG]
Overall, our project was well received and seemed to spark some serious interest. I think the simplicity of the concept combined with the potential widespread impact of our project is what was most appealing. We received many comments saying that not only would this type of bandage have impact in the developing world but also that it would be marketable in the United States. After only three weeks of work I really didn't expect this type of reaction. I feel that our demo was an important aspect of the presentation because people were able to see the color change within a matter of seconds. Even though there were no bacteria present, it convinced people that the colorimetric reaction actually worked.


